Magnets might not stick when trying to speed up medical tests, but that’s precisely what MagIC Lifescience claims to have done. A startup hailing from sunny California and led by seven experts. The company promises that its MagChipR lab-on-chip analyzer can reduce Polymerase Chain Reaction (PCR) test time to just 20 minutes with its giant magnetoresistance (GMR) biosensor.
Fig 1. The Magic Lifescience MagChipR system (under development).
The MagChipR is based on the principles of the standard PCR test, a common and accurate method for diagnosing a wide range of diseases and genetic changes, including sexually transmitted infections (STIs) and COVID-19. Nevertheless, PCR has its shortcomings, including potentially long wait times and the need for specialized equipment and trained technicians to perform it correctly.
Similar to a standard PCR test, the MagChipR begins by replicating or amplifying pieces of DNA in patient samples using different molecules until a threshold is reached. However, in contrast to a standard PCR test, which only detects genetic materials, the targeted DNA samples in the MagChipR undergo a binding process with other molecules until they attach to a magnetic nanoparticle, generating a magnetic field. It is by detecting this change in the magnetic field that the MagChipR can accurately tell if a pathogen is present.
Because the GMR biosensor is highly sensitive to magnetic field fluctuations, it needs less amplified materials to detect its target. Coupled with its proprietary ultra-fast PCR chemistry, the MagChipR can yield results in just 20 minutes. It can perform up to 64 tests simultaneously on a single chip using a variety of sample types, such as blood and plasma.
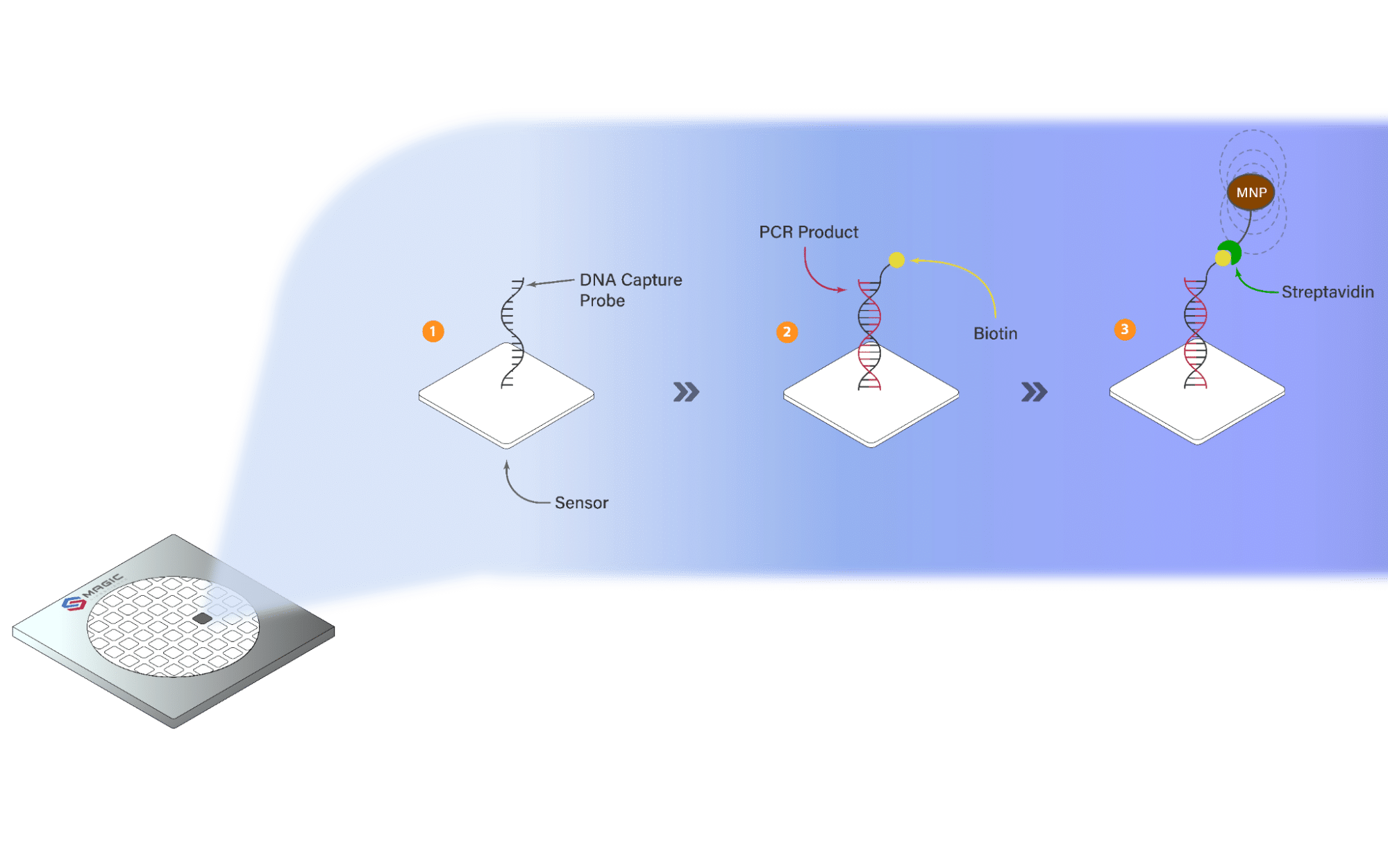
Fig 2. In the final stages of the binding process, biotin binds to the protein streptavidin, forming a strong bond. The streptavidin then attaches to the magnetic nanoparticle (MNP), changing the magnetic field to be detected by the GMR biosensor. Image source: MagIC Lifescience.
Bryce Yao, founder and chief executive officer of MagIC Lifescience, says that it currently can take five to 10 days to receive a PCR test result after the patient’s initial doctor visit. Traditional PCR tests need specialized lab equipment that are often only available at designated testing facilities, and transporting samples to these facilities can significantly delay testing. Moreover, the PCR tests need to be performed by skilled technicians.
In contrast, the compact MagChipR operates at the point of care, allowing samples to be immediately tested upon after they’ve been obtained from the patient. All tests in the MagChipR are done automatically; healthcare providers need minimal training to operate the device. In fact, Yao believes that the MagChipR is so easy to use, it may one day sit in people’s homes.
Cost is another bonus. In addition to promising highly accurate results, MagIC Lifescience also touts that the system can be easily manufactured. The technology is compatible with standard integrated circuit fabrication, and each cartridge costs as little as $5 to produce. Its affordability is directly tied to accessibility. Yao hopes that the MagChipR can increase quality of care in underserved communities and developing nations.
It can even be used outside of the clinic. Yao described a scenario where the MagChipR can help beauty salons identify their customers’ selected genotypes to recommend beauty products more suited for them.
Given its breadth of utility and potential, MagChipR has helped MagIC Lifescience attract over $5 million in funding, including support from the Bill & Melinda Gates Foundation.
For the moment, Yao wants to continue to optimize the technology to make it as safe and reliable as possible. To do so, MagIC Lifescience has planned a clinical trial involving 3,000 patients.
“Doctors are conservative providers, I think that’s necessary, because if you change something recklessly, people’s lives are at risk,” said Yao. “That’s why we have to do a lot of work in proving the efficacy and safety of our product that we’re going to [release] into the market, which translates into doing a lot of work in the clinical trials.”
MagChipR is currently preparing for FDA Clearance. Yao expects the device to be available on the market by the end of 2025.








